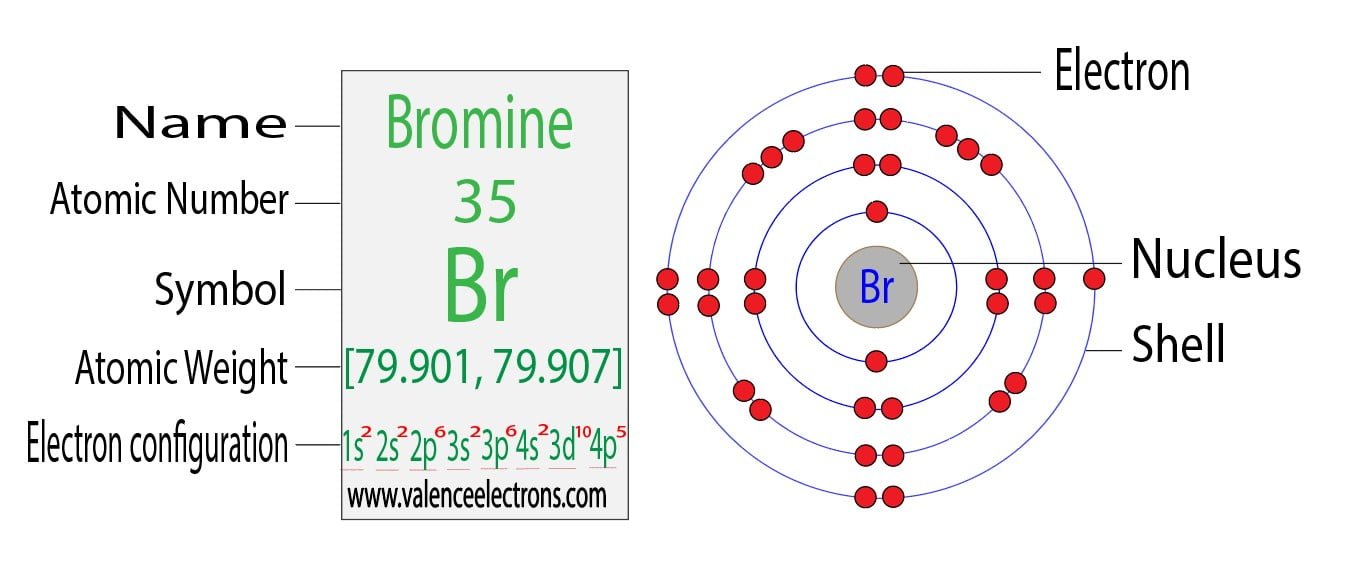
Bromine How Many Electrons . The atomic number for bromine is 35, which means it has 35 protons in its. the electron configuration of bromine shows that the last shell of bromine has seven electrons. It has an atomic weight of. Each electron is influenced by the electric fields produced by the positive nuclear charge and. Sources, facts, uses, scarcity (sri), podcasts, alchemical. You will see that the 3d sublevel is filled before the 4p after the 4s. bromine is the 35th element in the periodic table and has a symbol of br and atomic number of 35. therefore, the number of electrons in neutral atom of bromine is 35. bromine atoms have 35 electrons and the shell structure is 2.8.18.7. The ground state electronic configuration of neutral bromine is. The order of the filling is from bottom to top, that adds the electrons to many sublevels that are 1s, 2s, 2p, 3s, 3p, 4s, 3d, and 4p.
from www.animalia-life.club
Sources, facts, uses, scarcity (sri), podcasts, alchemical. The atomic number for bromine is 35, which means it has 35 protons in its. You will see that the 3d sublevel is filled before the 4p after the 4s. bromine atoms have 35 electrons and the shell structure is 2.8.18.7. The order of the filling is from bottom to top, that adds the electrons to many sublevels that are 1s, 2s, 2p, 3s, 3p, 4s, 3d, and 4p. therefore, the number of electrons in neutral atom of bromine is 35. the electron configuration of bromine shows that the last shell of bromine has seven electrons. Each electron is influenced by the electric fields produced by the positive nuclear charge and. The ground state electronic configuration of neutral bromine is. bromine is the 35th element in the periodic table and has a symbol of br and atomic number of 35.
Electron Configuration For Bromine
Bromine How Many Electrons Sources, facts, uses, scarcity (sri), podcasts, alchemical. It has an atomic weight of. The atomic number for bromine is 35, which means it has 35 protons in its. You will see that the 3d sublevel is filled before the 4p after the 4s. The ground state electronic configuration of neutral bromine is. therefore, the number of electrons in neutral atom of bromine is 35. the electron configuration of bromine shows that the last shell of bromine has seven electrons. bromine atoms have 35 electrons and the shell structure is 2.8.18.7. Each electron is influenced by the electric fields produced by the positive nuclear charge and. The order of the filling is from bottom to top, that adds the electrons to many sublevels that are 1s, 2s, 2p, 3s, 3p, 4s, 3d, and 4p. bromine is the 35th element in the periodic table and has a symbol of br and atomic number of 35. Sources, facts, uses, scarcity (sri), podcasts, alchemical.
From ar.inspiredpencil.com
Bromine Valence Electrons Bromine How Many Electrons The atomic number for bromine is 35, which means it has 35 protons in its. Each electron is influenced by the electric fields produced by the positive nuclear charge and. the electron configuration of bromine shows that the last shell of bromine has seven electrons. Sources, facts, uses, scarcity (sri), podcasts, alchemical. bromine is the 35th element in. Bromine How Many Electrons.
From www.animalia-life.club
Electron Configuration For Bromine Bromine How Many Electrons Sources, facts, uses, scarcity (sri), podcasts, alchemical. The atomic number for bromine is 35, which means it has 35 protons in its. You will see that the 3d sublevel is filled before the 4p after the 4s. bromine is the 35th element in the periodic table and has a symbol of br and atomic number of 35. bromine. Bromine How Many Electrons.
From www.animalia-life.club
Electron Configuration For Bromine Bromine How Many Electrons You will see that the 3d sublevel is filled before the 4p after the 4s. The order of the filling is from bottom to top, that adds the electrons to many sublevels that are 1s, 2s, 2p, 3s, 3p, 4s, 3d, and 4p. bromine is the 35th element in the periodic table and has a symbol of br and. Bromine How Many Electrons.
From www.dreamstime.com
Bromine stock illustration. Illustration of render, formula 139651101 Bromine How Many Electrons The order of the filling is from bottom to top, that adds the electrons to many sublevels that are 1s, 2s, 2p, 3s, 3p, 4s, 3d, and 4p. Each electron is influenced by the electric fields produced by the positive nuclear charge and. therefore, the number of electrons in neutral atom of bromine is 35. the electron configuration. Bromine How Many Electrons.
From periodictable.me
How Do We Find The Electron Configuration For Bromine Dynamic Bromine How Many Electrons The order of the filling is from bottom to top, that adds the electrons to many sublevels that are 1s, 2s, 2p, 3s, 3p, 4s, 3d, and 4p. It has an atomic weight of. The ground state electronic configuration of neutral bromine is. Sources, facts, uses, scarcity (sri), podcasts, alchemical. Each electron is influenced by the electric fields produced by. Bromine How Many Electrons.
From www.youtube.com
How many valence electrons does bromine have? YouTube Bromine How Many Electrons It has an atomic weight of. The ground state electronic configuration of neutral bromine is. You will see that the 3d sublevel is filled before the 4p after the 4s. therefore, the number of electrons in neutral atom of bromine is 35. the electron configuration of bromine shows that the last shell of bromine has seven electrons. The. Bromine How Many Electrons.
From sciencenotes.org
Bromine Facts Atomic Number 35 and Element Symbol Br Bromine How Many Electrons You will see that the 3d sublevel is filled before the 4p after the 4s. Each electron is influenced by the electric fields produced by the positive nuclear charge and. The order of the filling is from bottom to top, that adds the electrons to many sublevels that are 1s, 2s, 2p, 3s, 3p, 4s, 3d, and 4p. The atomic. Bromine How Many Electrons.
From periodictable.me
Bromine Electron Configuration (Br) with Orbital Diagram Bromine How Many Electrons The ground state electronic configuration of neutral bromine is. therefore, the number of electrons in neutral atom of bromine is 35. The order of the filling is from bottom to top, that adds the electrons to many sublevels that are 1s, 2s, 2p, 3s, 3p, 4s, 3d, and 4p. Each electron is influenced by the electric fields produced by. Bromine How Many Electrons.
From periodictable.me
Bromine Valence Electrons Bromine Valency (Br) Dot Diagram Bromine How Many Electrons the electron configuration of bromine shows that the last shell of bromine has seven electrons. bromine is the 35th element in the periodic table and has a symbol of br and atomic number of 35. The ground state electronic configuration of neutral bromine is. therefore, the number of electrons in neutral atom of bromine is 35. . Bromine How Many Electrons.
From www.youtube.com
How to find the Number of Protons, Electrons, Neutrons for Bromine (Br Bromine How Many Electrons the electron configuration of bromine shows that the last shell of bromine has seven electrons. Sources, facts, uses, scarcity (sri), podcasts, alchemical. You will see that the 3d sublevel is filled before the 4p after the 4s. therefore, the number of electrons in neutral atom of bromine is 35. bromine is the 35th element in the periodic. Bromine How Many Electrons.
From ar.inspiredpencil.com
Atomic Structure Of Bromine Bromine How Many Electrons The ground state electronic configuration of neutral bromine is. the electron configuration of bromine shows that the last shell of bromine has seven electrons. Each electron is influenced by the electric fields produced by the positive nuclear charge and. The atomic number for bromine is 35, which means it has 35 protons in its. The order of the filling. Bromine How Many Electrons.
From www.nuclear-power.com
Bromine Atomic Number Atomic Mass Density of Bromine nuclear Bromine How Many Electrons The order of the filling is from bottom to top, that adds the electrons to many sublevels that are 1s, 2s, 2p, 3s, 3p, 4s, 3d, and 4p. The ground state electronic configuration of neutral bromine is. You will see that the 3d sublevel is filled before the 4p after the 4s. bromine is the 35th element in the. Bromine How Many Electrons.
From www.vectorstock.com
Symbol and electron diagram for bromine Royalty Free Vector Bromine How Many Electrons The ground state electronic configuration of neutral bromine is. You will see that the 3d sublevel is filled before the 4p after the 4s. It has an atomic weight of. bromine is the 35th element in the periodic table and has a symbol of br and atomic number of 35. Each electron is influenced by the electric fields produced. Bromine How Many Electrons.
From www.schoolmykids.com
Bromine (Br) Element Information, Facts, Properties, Uses Periodic Bromine How Many Electrons bromine atoms have 35 electrons and the shell structure is 2.8.18.7. The ground state electronic configuration of neutral bromine is. Sources, facts, uses, scarcity (sri), podcasts, alchemical. It has an atomic weight of. Each electron is influenced by the electric fields produced by the positive nuclear charge and. The atomic number for bromine is 35, which means it has. Bromine How Many Electrons.
From exobbalij.blob.core.windows.net
Bromine Atom Electrons at Paula McCullough blog Bromine How Many Electrons The ground state electronic configuration of neutral bromine is. the electron configuration of bromine shows that the last shell of bromine has seven electrons. The order of the filling is from bottom to top, that adds the electrons to many sublevels that are 1s, 2s, 2p, 3s, 3p, 4s, 3d, and 4p. You will see that the 3d sublevel. Bromine How Many Electrons.
From www.schoolmykids.com
Bromine (Br) Element Information, Facts, Properties, Uses Periodic Bromine How Many Electrons The ground state electronic configuration of neutral bromine is. It has an atomic weight of. the electron configuration of bromine shows that the last shell of bromine has seven electrons. therefore, the number of electrons in neutral atom of bromine is 35. The order of the filling is from bottom to top, that adds the electrons to many. Bromine How Many Electrons.
From srkrkzbhxtdlh.blogspot.com
How Many Valence Electrons Does Bromine Have Bromine is a group viia Bromine How Many Electrons therefore, the number of electrons in neutral atom of bromine is 35. The order of the filling is from bottom to top, that adds the electrons to many sublevels that are 1s, 2s, 2p, 3s, 3p, 4s, 3d, and 4p. the electron configuration of bromine shows that the last shell of bromine has seven electrons. The atomic number. Bromine How Many Electrons.
From www.slideserve.com
PPT Topic Chemistry Aim Explain how elements are classified in Bromine How Many Electrons bromine is the 35th element in the periodic table and has a symbol of br and atomic number of 35. The ground state electronic configuration of neutral bromine is. Each electron is influenced by the electric fields produced by the positive nuclear charge and. Sources, facts, uses, scarcity (sri), podcasts, alchemical. the electron configuration of bromine shows that. Bromine How Many Electrons.